Risk Evaluation and Mitigation Strategies (REMS) is a drug safety program required by the US FDA for certain medicines that are associated with safety concerns. They typically accompany applications to the US FDA, like New Drug Applications, Biologics License Applications, or Abbreviated New Drug Applications (ANDA), that describe drug safety and ensure that the drug’s benefits outweigh its risks. REMS is implemented under the FDA Amendments Act of 2007, to manage known or potentially serious risks associated with certain drugs, particularly those that carry significant safety concerns. Each REMS program is tailored to the specific medication and its associated risks, incorporating elements such as a medication guide, communication plans, and Elements to Assure Safe Use (ETASU). REMS are usually not required for all drugs; they are implemented only when the FDA determines that additional safety measures beyond prescribing information are necessary. This strategy aims to enhance safe medication use, focus on preventing, monitoring, and managing risks, enhancing patient safety, and reducing adverse events.
Components of REMS
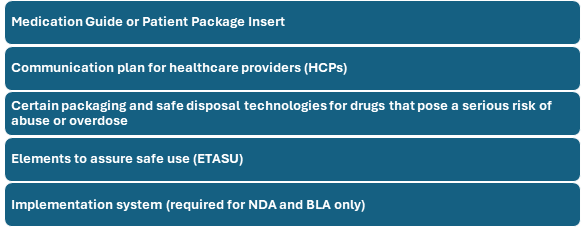
The key elements of a REMS program can vary based on the drug’s risk profile, but generally include the following:
Impact of REMS on Generic Drug Development
A study conducted by the U.S. Government Accountability Office (GAO) reports that generic drug manufacturers face several challenges as generic manufacturers often need samples of reference drugs to conduct bioequivalence studies. REMS with ETASU can restrict access to these samples, making it difficult for generic applicants to obtain the requirements for testing. According to the FDA and the Federal Trade Commission (FTC), brand-name companies may use REMS requirements as a basis for delaying or restricting access to samples, leading to legal disputes and delays in the drug development process.
To overcome these challenges, the FDA developed a single, shared system REMS (SSSRs). SSSRs are designed to allow multiple sponsors, including both brand-name and generic drug manufacturers, to work together under a unified REMS framework. This approach aims to benefit applicants and sponsors, simplifying safety data collection and analysis, reducing administrative burden for prescribers and pharmacists, and providing sponsors with opportunities to share program development costs.
Legislative Efforts and Proposals:
- The FAST Generics Act of 2017 aims to prevent brand companies from using REMS to block generic drug development by ensuring access to necessary samples and negotiating shared REMS systems more effectively.
- The CREATES Act of 2017 proposes mechanisms to facilitate generic sample availability and streamline shared REMS system development, including civil action against withholding samples.
Developing a Single, Shared System REMS
A Single, Shared System REMS is required for both branded and generic drugs with ETASU to streamline safety protocols and reduce the burden on healthcare providers and patients. The process involves the FDA notifying the generic drug manufacturer to collaborate with the brand-name company to create a shared system. This requires negotiations on cost-sharing, safety protocols, and intellectual property issues. The FDA facilitates the process, ensuring both parties work towards a common goal, with periodic updates and reviews. While the aim is to reduce the burden on stakeholders, challenges such as delays in negotiations and the need for waivers in some cases persist. Legislative efforts like the CREATES Act and the FAST Generics Act seek to streamline this process and prevent delays caused by brand name companies.
The SSS REMS provides benefits by promoting efficiency, reducing costs, enhancing data analysis capabilities, improving communication, and simplifying regulatory compliance.
The US FDA has proposed guidance for the format and content of REMS. A successful REMS submission should include two parts: the REMS (REMS document and REMS materials) and the supporting document. A proposed REMS can be submitted with the initial application (NDA, ANDA, or BLA) or as an amendment to an existing application. All approved REMS documents and materials are published on the FDA’s website.
Conclusion
By collaborating among brand-name and generic drug manufacturers, shared REMS can streamline the process of risk management, reduce costs, fasten the process, and enhance patient safety. As the US pharmaceutical sector is evolving rapidly, stakeholders need to work together to speed up the drug development process.
If you have any REMS-related queries or need expert guidance on navigating the complexities of risk management strategies, connect with our team of experts today.
In addition to providing regulatory and pharmacovigilance services for global customers, DDReg Pharma offers specialized clinical regulatory services to assist with Clinical Trial Applications in the United States. For further information, connect with our experts. Read more about REMS regulations from the experts here: FDA Launches REMS Public Dashboard to Enhance Transparency and Data Access

