Africa’s healthcare and regulatory landscape have transformed to reflect the commitment of the continent to improve public health and ensure the safety and efficacy of medical products. Understanding this evolution is important for pharmaceutical manufacturing firms and professionals seeking regulatory services to navigate the African market efficiently.
Historical Context
The history of health in Africa is shaped by the political developments of the continent. Health outcomes have been moulded by social, economic, environmental and political conditions from precolonial to contemporary times. We need an integrated study of African histories of health in order to understand the dynamic interaction of these factors.
In the mid-20th century, a number of African states became independent and declared health a human right. However, the economic difficulties of the 1980s introduced Structural Adjustment Programs (SAPs), which brought a new funding system with decreased government healthcare spending. During this period, free health care is removed, affecting both access and quality.
Regulatory Evolution
Recognizing the need for robust regulatory systems, African nations have made concerted efforts to harmonize and strengthen their regulatory frameworks. The establishment of the African Medicines Agency (AMA) marks a significant milestone in this journey. The AMA aims to enhance regulatory harmonization across the continent, ensuring that medical products meet consistent standards of quality, safety, and efficacy.
Additionally, initiatives like the African Medicines Regulatory Harmonization (AMRH) have been instrumental in aligning regulatory processes, reducing duplication, and expediting the approval of essential medicines. These efforts have been supported by regional collaborations and the development of model laws to guide national regulatory practices.
Regulatory Milestones
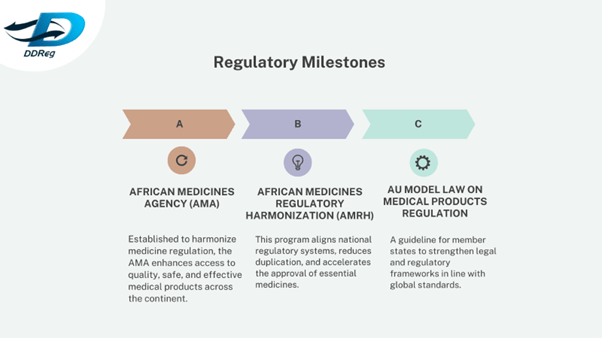
These milestones signify Africa’s commitment to ensuring the safety and efficacy of medical products.
Current Landscape and Opportunities
The pharmaceutical regulatory affairs market in Africa is undergoing rapid growth. The global market worth USD 126.04 million in 2022, is projected to grow at a compound annual growth rate (CAGR) of 6.81% during 2023 to 2030. Factors such as the growing prevalence of chronic diseases; increasing elderly population; rising healthcare spending; and the promoter of innovative and cost-effective medicines throughout the region, are driving this growth.
There is also a strong focus on the local manufacture of pharmaceuticals. Having regional pharmaceutical manufacturing hubs can lead to increased access to drugs, reduced costs and enhanced health systems in those regions. This shift would ideally lessen reliance on imports and enable greater access to essential drugs.
Challenges in the Healthcare Sector
Despite much progress, the healthcare sector still has several challenges to overcome:
- Workforce Shortages: The continent suffers from an alarming shortage of health professionals, putting more burden on the already overwhelming health systems. Some countries have less than 1 doctor per 1,000 people.
- Resource Gap: It remains a common problem for many states to provide their national healthcare infrastructures and services with appropriate funding and staffing through enshrined declarations like the Abuja Declaration.
- Fragmented Systems: The differences in regulatory standards across countries create considerable complexities for the suppliers entering African markets.
Opportunities for Growth
The transformation of Africa’s healthcare system offers novel opportunities for pharmaceutical manufacturers:
- Expand local manufacturing – initiatives like the Pharmaceutical Manufacturing Plan for Africa seek to increase the local production of medicines and thus reduce reliance on imports.
- Heightened Digital Health Investment: Devices like telemedicine, mobile health (mHealth), as well as digital patient files are transforming the distribution of health care.
- Universal Health Coverage (UHC): Community-based health insurance schemes provide access to health care across regions.
Implications for Pharmaceutical Manufacturers
To capitalize on these opportunities, manufacturers must:
- Stay informed about regulatory updates and harmonization efforts.
- Partner with local stakeholders to navigate market complexities.
- Participate in capacity-building programs to foster mutual growth and compliance.
Conclusion
Africa’s regulatory and healthcare landscape is undergoing a profound transformation, offering new horizons for pharmaceutical firms. With harmonized frameworks and technological advancements, the continent is poised to bridge healthcare gaps.
DDReg can help your organization contribute to this transformative journey. Partner with our regulatory experts today to explore and navigate the promising African healthcare market today. Read more from us here: A Strategic Approach for Managing Pharma Regulatory Risks with Big Data

