Quality by design is a systematic approach to pharmaceutical development that begins with a predetermined objective and emphasizes product and process understanding and process control based on science and quality risk management. This method is redesigned to improve drug safety, efficacy, and overall quality by systematically identifying and managing critical quality attributes (CQAs) and critical process parameters (CPPs). A core aspect of this QbD, which is now mandated by regulatory authorities such as the FDA and EMA, requires that the system be designed to ensure the quality is “built-in” rather than tested for at the end of the production process. QbD focuses on understanding the link between CQAs and CPPs. CQAs are measurable characteristics that define how a product performs, whereas CPPs are the factors that influence the manufacturing process. Let’s understand the implementation of Quality by Design (QbD) Approach in Regulatory Submissions.
Key elements of QbD in Regulatory Affairs
Steps to Implement QbD in Regulatory Submissions
When incorporating Quality by Design (QbD) into regulatory submissions, it includes an overarching strategy of pharmaceutical development and production. The intention is to avoid any uncertainty regarding the product and the process which will ultimately lead to a product of the desired quality and adherence to all the relevant expectations.
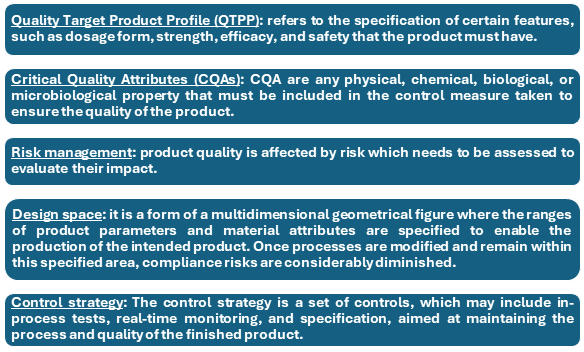
Establish Quality Target Product Profile (QTPP): Identify Desired Attributes: Initiate with the QTPP, it serves to direct product development and also ensures compliance with therapeutic and regulatory claims. Factors such as regulatory and patient requirements should be included while describing the QTPP.
Identify Critical Quality Attributes (CQAs); Identify the CQAs that impact the product’s safety, efficacy, and quality. CQAs are defined or measurable features that have to be controlled within pre-defined limits to ensure the end product’s processed attributes. Conduct a risk assessment to determine which attributes are critical. This assesses the variability of these attributes and the quality of the product.
Conduct Risk Assessments: Apply Quality Risk Management (QRM) tools, such as Failure Mode and Effects Analysis (FMEA) or Hazard Analysis and Critical Control Points (HACCP), to identify and control risks related to CQAs and process parameters. Assess the risks that are identified during the assessment and formulate mitigation measures to control them. This ensures that stakeholders are not left with any critical risk unattended.
Establish Design Space: Employ Design of Experiments (DOE) to study systematically the effect of process parameters on CQAs. This helps to determine a design space in which the process can operate without malfunctioning.
Develop Control Strategy: Formulate a control strategy for CQAs monitoring at all the stages of the production process to maintain the parameters within acceptable levels. This includes in-process controls, real-time monitoring, and quality control tests.
Use Process Analytical Technology (PAT) tools to keep track of the critical elements in real-time and to adjust as appropriate to maintain the quality.
Document QbD Elements: The QbD elements, including the QTPP, CQAs, risk assessments, design space, and control strategy, must be documented as they are integral to any regulatory filings.
Continuous Improvement and Lifecycle Management: Implement a continuous monitoring system for process improvement, regularly reviewing performance and adjusting based on new data. Adopt a lifecycle approach to quality management, ensuring products and processes meet quality standards throughout their lifecycle.
Engage with Regulatory Agencies: Engage with regulatory agencies early in the development process to discuss the QbD approach and gather feedback, aligning the strategy with regulatory expectations.
ICH Guidelines for QbD
QbD is supported by ICH guidelines, which provide a harmonized framework for pharmaceutical quality. These guidelines emphasize understanding product and process development, quality risk management, and a comprehensive quality system model that integrates QbD principles throughout the product lifecycle.
ICH Q8 (R2) (Pharmaceutical Development): Provides a comprehensive framework for implementing Quality by Design in pharmaceutical development
ICH Q9(R1) (Quality Risk Management): Provide guidance on implementing risk management principles to identify and mitigate potential quality risks.
ICH Q10 (Pharmaceutical Quality System): Provides a comprehensive framework for establishing and maintaining an effective Pharmaceutical Quality System (PQS) throughout the product lifecycle.
Conclusion
Implementing a Quality by Design (QbD) approach in regulatory submissions is not just a regulatory requirement; it is a strategic advantage that can significantly enhance product quality and streamline the development process. QbD generates a further understanding of a product, reduces risks, and expands the robustness of regulatory flexibility, resulting in a much higher quality product and potentially easier approval. By incorporating all QbDs the companies would comply with global regulatory requirements and promote innovation efficiently throughout the product life cycle.
In addition to providing regulatory and pharmacovigilance services for global customers, DDReg Pharma offers specialized clinical regulatory services to assist with Clinical Trial Applications in the United States. For further information, connect with our experts. Read more about USFDA regulations from the experts here: Key Insights from FDA’s ICH M13A Bioequivalence Guidelines

