In recent years, the emphasis on diversity in clinical trials has become more prominent, reflecting the need for studies on more diverse populations to gather more accurate clinical findings. The lack of diversity in participants can create an obstacle to understanding the safety and efficacy of new medicines, making it more difficult to understand the side effects. This information gap could result in health disparities, with certain groups experiencing less efficacious treatment or more adverse effects. It is essential that sponsors should try to include people with various characteristics like age, gender, race, ethnicity, disease experiences, etc to ensure they have covered the population in question and mitigate risks of underrepresentation in trials.
Recognizing the diversity in population is important for clinical research, as it ensures that clinical trials (CTs) accurately reflect variation among individuals. Diversifying CTs helps in understanding the demographics’ response to treatments.
Variability in treatment response: People from different ages, gender, race, ethnicity, and socioeconomic backgrounds respond differently to diseases and their treatments due to genetic, environmental, and lifestyle factors.
Regulatory requirements: Regulatory agencies are now mandating that clinical research include diverse populations. The protocol and study report must contain the inclusion and exclusion criteria for selection.
Ethical considerations: Today, all clinical studies must follow Good Clinical Practice (GCPs). As part of Good Clinical Practice, institutional review boards (IRBs) or independent ethics committees (IECs) supervise CTs.
Challenges and Barriers to Achieving Diversity in Clinical Trials FDA
While the importance of diversity in CTs is widely recognized, several significant challenges and barriers may hinder the effective inclusion of diverse populations. Addressing these challenges is crucial to ensuring that clinical research benefits all segments of society.
For example, the lack of trust of minorities in the medical community is the major barrier. Overcoming this mistrust requires sustained efforts to build trust through community engagement, transparency, and ethical clinical research practices. Furthermore, potential participants are unaware of the conduct of clinical trials and their procedures. Educational campaigns and advertisements through various forms of media that explain the benefits and safety measures of participating in clinical research to address this. Lastly, clinical trial staff must be culturally competent and speak the participant’s native language.
The image below shows the scenario of diversity and inclusion in clinical trials from a “regulation vs reality” point of view.
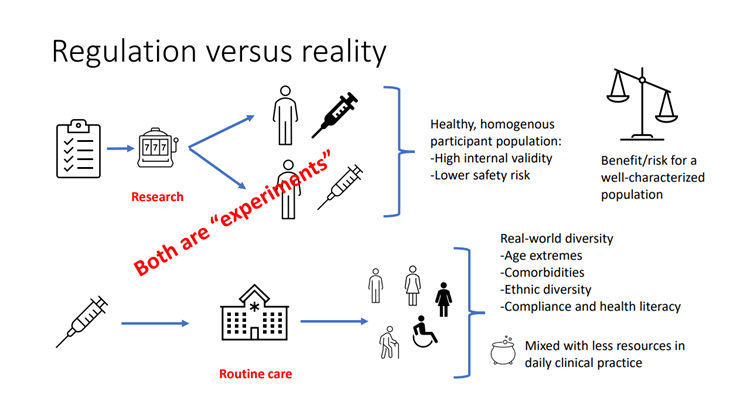
Source: Diversity and inclusion in clinical trials – a scientific need and a moral obligation
Regulatory Changes and New Requirements for Diversity in Clinical Trials
In November 2020, the US FDA introduced guidelines and requirements to enhance diversity in clinical research by issuing a guidance document on “Enhancing the Diversity of Clinical Trial Populations – Eligibility Criteria, Enrollment Practices, and Trial Designs”.
The key objectives of the guidance were:
- To broaden the eligibility criteria by adopting various trial designs and methodological approaches.
- To implement enrichment strategies for broadening eligibility criteria
- To adopt more inclusive enrolment practices
In June 2024, the FDA introduced “Diversity Action Plans to Improve Enrollment of Participants from Underrepresented Populations in Clinical Studies” guidance, with a focus on increasing clinical trial enrolment for underrepresented populations. The Guidance mainly focuses on age, sex, racial and ethnic demographic factors, but also encourages considering other aspects of diversity, such as geographic location, gender identity, sexual orientation, socioeconomic status, physical and mental disabilities, pregnancy status, lactation status, etc.
Clinical research that necessitates Diversity Action Plan
Under sections 505(z) and 520(g)(9) of the FD&C Act, the FDA recommends sponsors submit a Diversity Action Plan (DAP) for Phase 3 studies of new drugs or pivotal clinical studies, and any clinical study of investigational devices unless they qualify for an IDE exemption under 21 CFR 812.2(c).
DAP must include the following:
Enrolment goals: it must be disaggregated by race, ethnicity, sex, and age groups of clinical participants.
The rationale for enrolment goals: justification for choosing the enrolment goals, including the background information on the disease for which the drug or devices are being tested.
Measures to meet enrolment goals: The final DAP must outline plans to meet enrollment goals, including strategies like community engagement, cultural competency training, language assistance, transportation assistance, flexible hours, limiting study exclusion criteria, selecting sites with diverse populations, and decentralized design.
Procedures and Timeline for DAP Submission
Drugs: Sponsors must submit DAPs to the FDA no later than the date of the Phase 3 study protocol submission. However, the FDA encourages earlier submissions, such as at the End-of-Phase 2 meeting. DAPs must be submitted to the investigational new drug application (IND) under which the clinical study will be conducted.
Devices: DAPs must be included in the Investigational Device Exemption (IDE) application. For studies without an IDE application, sponsors must submit the DAP as part of the 510(k), device’s premarket notification, PMA application, or De Novo classification request.
Conclusion
Navigating new requirements for diversity in clinical research is not merely a regulatory requirement but also a pivotal step in gathering more information on the disease and its treatment. The gaps will be narrower with the inclusion of more diverse populations including women and minorities. Prioritizing diversity not only enhances drug-disease knowledge but also builds trust among minorities that have been underrepresented historically. The success of clinical research hinges on sticking to the new regulatory requirements and diversifying the study participants.
In addition to providing regulatory and pharmacovigilance services for global customers, DDReg Pharma offers specialized clinical regulatory services to assist with Clinical Trial Applications. For further information, connect with our experts. Read more from the experts about Clinical Trial Information here: Faster Access to Clinical Trial Information in Europe

