Vaccines are among the greatest achievements of modern medicine, safeguarding millions from infectious diseases. With the rapid evolution of global healthcare needs, particularly during pandemics, vaccine regulations have become more critical than ever. Both the United States and the European Union (EU) maintain stringent frameworks to ensure the safety, efficacy, and availability of vaccines. While these frameworks share common principles, they also reflect unique regional priorities and regulatory landscapes.
Vaccine development is a lengthy and intricate process, often spanning years and involving multiple phases of research and testing. From exploratory and preclinical stages to phased clinical trials and eventual licensure, the journey of a vaccine involves meticulous scrutiny to ensure its safety and effectiveness. Regulatory bodies in both Europe and the U.S. play a critical role in overseeing this process, balancing public health needs with scientific rigor. Let’s understand the Regulatory Framework for Vaccines in United States and Europe.
Regulatory Framework for Vaccines in United States
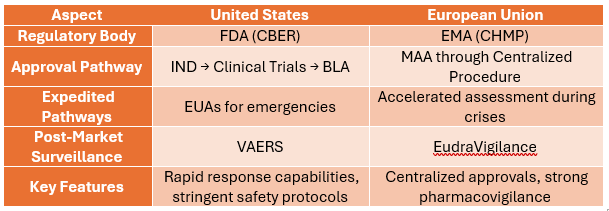
In the USA, vaccine regulation falls under the scope of the Center for Biologics Evaluation and Research (CBER), a branch of the Food and Drug Administration (FDA). Furthermore, the Vaccines and Related Biological Products Advisory Committee (VRBPAC) review and assess data regarding vaccines. The approval process typically includes:
- Investigational New Drug (IND) Application
Before clinical trials, sponsors must file a IND application detailing preclinical data, manufacturing processes, and clinical trial protocols.
- Clinical Trials
- Phase I: Tests safety and immune response in a small group of participants.
- Phase II: Expands participant numbers to evaluate immunogenicity and identify side effects.
- Phase III: Involves thousands of participants to confirm efficacy and monitor rare adverse reactions.
Post successful clinical trials, sponsors submit a BLA, which undergoes priority (6 months) or standard review (12 months). Key components include:
- Data on manufacturing and controls
- Clinical trial results
- Risk assessment
Once approved, the product enters Phase IV (post-marketing surveillance) to monitor ongoing safety and effectiveness through systems like the Vaccine Adverse Event Reporting System (VAERS).
Regulatory Framework for Vaccines in Europe
The EU adopts a collaborative approach under the guidance of the European Medicines Agency (EMA) and its advisory body, the Committee for Medicinal Products for Human Use (CHMP). National Competent Authorities of Member States have a role to play in mutual recognition or decentralized procedures.
Manufacturers generally prefer the centralized procedure, allowing vaccines to be approved for use across all EU member states.
- Clinical and Quality Assessment
Similar to the U.S., the EU mandates rigorous preclinical and clinical evaluations. Additionally, it employs the Vaccine Antigen Master File (VAMF) system, which streamlines documentation for multi-antigen products.
- Pharmacovigilance and Risk Management
Post-authorization, vaccines are monitored via the EudraVigilance system, ensuring timely identification of adverse events. Manufacturers must submit risk management plans (RMPs) and comply with stringent pharmacovigilance requirements.
Comparative Insights
Challenges and Future Trends
- Pandemic Preparedness: Accelerated frameworks, such as Emergency Use Authorizations (EUAs) in the U.S. and conditional marketing authorizations in the EU, have reshaped the regulatory landscape.
- Harmonization Needs: Greater alignment between U.S. and EU regulations could streamline global access. The US typically emphasizes on centralized control, domestic clinical trial data, and scientific advisory committees for decision marking. The EU’s approach is more on collaborative decision-making with EU Member States and broader acceptance of international trial data.
Conclusion
For pharmaceutical manufacturers, understanding these frameworks is paramount. Whether entering the U.S. market with its focused, structured approach or the EU’s comprehensive yet flexible system, thorough preparation ensures smoother pathways to approval.
Leverage DDReg’s expertise in regulatory affairs to accelerate your vaccine’s journey to market. Connect with us today to ensure compliance and success in the global healthcare arena.
Read more from us here: Medical Device Regulations in the United States

