The pharmaceutical industry is highly regulated using legislations and regulations that bring together administrative, technical, and legal information about a medicinal product.
These can be a combination of guidelines, procedures, policies, etc., that pharmaceutical organizations must adhere to, or follow, to get market approval for their products.
This way, governments and their relative health authorities can ensure that all medicinal products are safe, efficacious, and of high quality in the interest of public health.
An important means by which governments safeguard the medicinal products and protect public health is through regulatory evaluation that ensures the products being supplied meet the safety, efficacy and quality requirements for the sake of patient safety.
The organizations like TOPRA (The Organization for Professionals in Regulatory Affairs) and RAPS (regulatory Affairs Professional Society) are examples of an organization created to support regulatory affairs professionals. These organizations outline some of the key roles of regulatory professionals:
- Staying updated with any changes in legislations for regions in which organizations intend to obtain market access
- Providing advice on legal and scientific restraints and requirements
- Collecting, collating, and evaluating scientific/technical data
- Liaising with regulatory agencies to either obtain or renew marketing authorization for a product
- Maintaining regulatory records
Regulatory affairs throughout the product lifecycle
Pharmaceutical organizations have their pharmaceutical regulatory affairs team and/or they outsource regulatory services. These regulatory services teams get involved from early stages of drug development to post-marketing commercializing. For example, in research stages of drug development, regulatory affairs assist in developing a clinical development strategy that consists of carefully planned clinical trials from phase I to phase III or planning exhibit batches as per stability zones. This is important as any delays or errors in clinical trials can negatively affect the commercial prospects of the drug and cause delays in generating revenue.
Regulatory affairs in pharma help to develop detailed modules from 2 to 5 of the of the common technical document (CTD).
The reg affairs team frequently liaises with competent authorities (CAs) and agencies like FDA/EMA/UK MHRA/Australian TGA/ S African SAHPRA/ Health Canada etc to ensure that the information in the modules meet the requirements set by regulators, to ultimately ensure market access.
Chemical Manufacturing and Control (CMC) experts and the regulatory affairs team ensure that the CTD modules and compliantly compiled and submitted to the relevant authorities.
The regulatory affairs team facilitates a more efficient and timely product commercialization process by developing a detailed submission strategy for the marketing authorization dossiers that accounts for a region’s approval time, dossier requirements, registration route, and re-imbursement costs.
Regulatory Affairs team is the last but most important milestone in the product life cycle as it interfaces with CAs for obtaining the MAs.
Once the regulatory authority grants market approval for the product, there is additional regulatory work that needs to be done.
These include updating administrative, quality and/or safety information in the dossier for a product that has been authorized and registered.
These changes are called variations and are classified into type 1A, 1B, II in EU, UK markets or called as “Changes being effected” (CBE) in the US.
Additionally, the regulatory affairs team may be required to make new filings for existing dossiers in new markets of interests, or additional strengths may need to be added at later stages.
Upon completion of clinical trials, new indications may be identified for a medicine that requires the regulatory affairs team to submit a new marketing authorization application.
Switches in product classification from Rx to OTC drugs may be necessary thus requiring comprehensive knowledge on regulations for the switch, and the associated application to the relevant authorities.
Conclusion
The role of the regulatory affairs team is extremely important in the highly regulated pharmaceutical industry to ensure patient safety.
Involving regulatory experts with their extensive knowledge and experience during all product development stages is necessary to make sure that pharmaceutical products that are being consumed are safe, efficacious, and of high quality.
DDReg pharma offers comprehensive solution as part of its Regulatory Services portfolio. We have a track record of managing regulatory compliances for over 2,000 licenses globally and have delivered over 200 market authorizations in the EU and US.
We have also delivered over 600 marketing authorizations in emerging markets including the GCC, Africas, LatAm, CIS, & ASEAN.
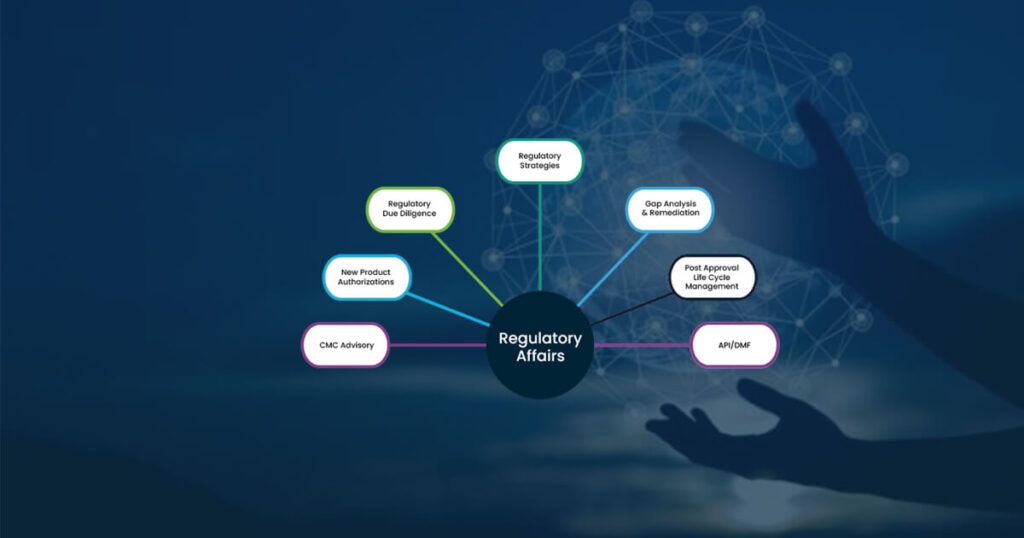
Our regulatory affairs services include global CMC support, post approval life cycle management, pre-authorization regulatory strategies and operations, regulatory due diligence, labeling management, global eCTD publishing and submission management, regulatory intelligence including data management and processing, medico-regulatory writing, and IPR support.