A Signal Management in Pharmacovigilance is information that arises from single or multiple source/s that may suggest a potential causal association between a drug and event given that the causal relationship between drug & event were previously unknown or inadequately documented. These signals are used for risk-benefit evaluation of a product and emerging safety issues that would cause harm to a patient. Usually, more than a single Individual Case Study Report (ICSR) is required to generate a signal.
Why is Signal Management Important in Pharmacovigilace?
Signal management processes are necessary for the early detection of unknown adverse events of medicines, for identifying early warnings of adverse events (serious & non-serious), to determine new risks and existing benefit-risk balance of medicinal products, and to determine what regulatory actions to take to minimize patient risk.
Steps Involved in Signal Management in Pharmacovigilance
Steps involved in Signal Management in Pharmacovigilance process include detection of signal, its validation, confirmation, evaluation/assessment and recommendation for action.
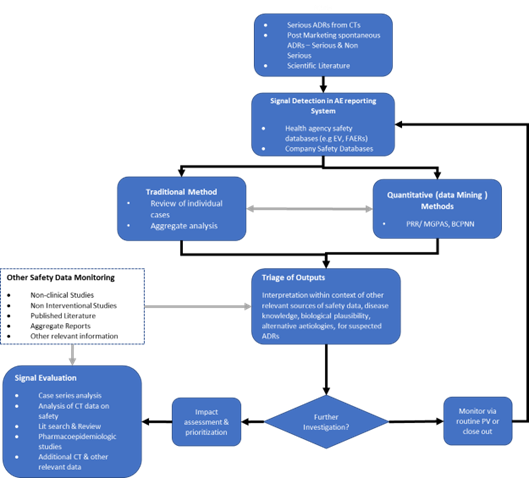
Pharmacovigilance Signal Management Process
Signal detection involves review of individual case safety reports, aggregate reports, scientific literature, agency website and use of quantitative statistical methods or a combination of these. Signals may arise from disparate sources including:
- Unsolicited sources like Spontaneous Reports, Literature, Social Media, Internet etc
- Solicited sources – Clinical trials, Registries, Epidemiology studies, compassionate use programs, Surveys, outcome research, Post Approval Safety Studies (PASS) etc that are organized data collection systems
- Agreements – Safety Data exchange agreements (SDEAs) between companies
- Regulatory Agency resources – FDA Adverse Event Reporting System (FAERS), EudraVigilance, VigiBase, Pharmacovigilance Risk Assessment Committee (PRAC)
To validate a signal, it is important to establish the fact that the data & evidence collected that supports the signal detected has sufficient evidence that demonstrates a pure causal relationship without any confounding factor. Any evidence of previous awareness, temporal & biological relationships etc should be duly considered while validation a signal. Regulatory agencies like EMA has repository like Pharmacovigilance Issues Tracking Tool (EPITT) which has been developed by EMA to enter validated signals & communicate the pharmacovigilance and risk management issues between the EMA and Member States. Signals for which the validation process did not point any causal association, or a new aspect of a known association, are not entered in EPITT. A validated signal has to be reviewed and assessed scientifically with all the evidences available.
It is important to implement a robust PV signal management system that is proactive and vigilant in detecting and managing safety signals. Regulatory authorities are increasingly placing stringent requirements for the detection and management of safety signals in order to mitigate patient risk and as a result life sciences organizations face cases of non-compliance as they lack a robust establishment required for effective signal management.

