In the rapidly evolving pharmaceutical landscape of the world, it is regulatory standards that ensure safe and effective drugs are delivered to patients. In Latin America, Brazil’s ANVISA has slowly been emerging as among the leading authorities of the region to help develop a more robust and globally harmonized framework. It stood out especially with recent developments in regulatory practice by setting up the Equivalent Foreign Regulatory Authority, AREE. According to ANVISA, the AREE is a “foreign regulatory authority or international organization with regulatory practices aligned with ANVISA’s, which is responsible for ensuring that the products authorized for distribution were appropriately assessed and meet recognized standards of quality, safety, and efficacy, and which shall be considered by ANVISA in a practice of regulatory reliance.”
The advent of AREE represents a significant change in how Brazil addresses pharmaceutical regulation, enabling reliance on a rather more intensified level of evaluation work done by foreign authorities well entrenched in developing economics. This contributes towards accelerating the approval procedure for new drugs and medicinal devices and places Brazil at a better standing with world standards. Historically, the overall regulatory environment in Brazil has been characterized by lengthy approval processes as well as administrative hurdles. The introduction of AREE aims to address this issue by leveraging the credibility of established foreign regulatory authorities.
Recently, ANVISA introduced two instructions:
Instructions no. 289/2024: with a focus on pharmaceutical products, biological products, and vaccines. It aims to streamline the evaluation process for these products by allowing ANVISA to rely on evaluations conducted by recognized AREE.
Instructions no. 290/2024. pertains to specially to medical devices, establishing similar protocols for their assessment and approval based on foreign evaluation.
Implications for pharmaceutical companies
The recent updates present benefits for pharma companies looking to enter or expand the Brazilian market.
Faster market access: By utilizing AREE assessment, companies will now be able to reduce approval times. This expedited process allows for faster access to a growing market.
Increased efficiency: The reliance on foreign regulatory assessment means that companies can streamline their regulatory submissions. Instead of duplicating efforts to meet local regulatory requirements, they can leverage existing approvals from recognized AREEs.
Attracting Global Pharma Companies: The streamlined processes and reduced regulatory barriers make Latin America a more attractive destination for global pharmaceutical companies. This leads to increased investment in the region, including in local manufacturing, research, and development.
Improved Patient Outcomes: Patients in Latin America will benefit from quicker access to the latest drugs. This is crucial for conditions with unmet medical needs, where new drugs can improve patient outcomes and quality of life.
However, to qualify for this streamlined process, manufacturers must provide comprehensive documentation proving prior approval from designated AREE.
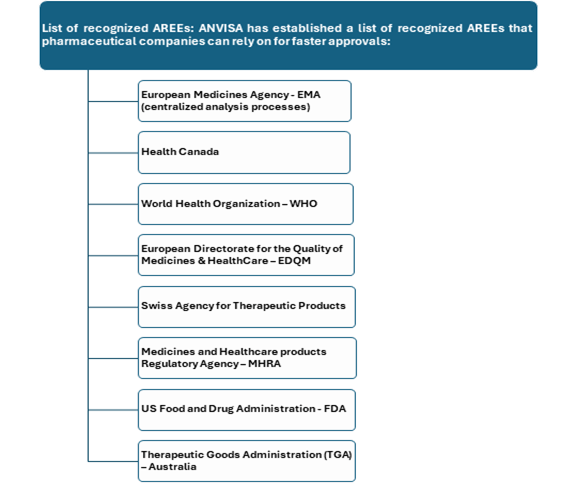
To be designated as an AREE, a foreign authority must demonstrate robust requirements practice that aligns with ANVISA standards. This recognition fosters trust and facilitates smoother collaboration between international firms and ANVISA.
Futuristic Vision
The recent changes in AREE regulations are expected to have a transformative impact on the pharmaceutical landscape in Latin America. As Brazil becomes more attractive due to the streamlined process, foreign investment in local pharmaceutical operations will likely rise. The AREE assessment signifies a novel move towards harmonizing the Brazilian regulatory framework with global practices, potentially leading to more collaborative efforts across borders.
Conclusion
The AREE update presents both opportunities and challenges for pharmaceutical companies operating in Latin America. The AREE framework has been updated to align Latin America’s regulatory environment with international standards, aiming to accelerate drug approval times, increase regulatory predictability, and open new market opportunities for pharmaceutical companies. These changes will drive sustainable growth in the pharmaceutical sector and attract long-term investments. Pharmaceutical companies must proactively engage with the updated framework, leveraging streamlined processes and investing in the region’s potential. Embrace the AREE updates and seize the opportunity to innovate and expand in Latin America. Together, let’s drive growth, enhance public health, and position Latin America as a leader in the global pharmaceutical landscape.
In addition to providing regulatory and pharmacovigilance services for global customers, DDReg Pharma offers specialized clinical regulatory services to assist with Clinical Trial Applications in Latin America. For further information, connect with our experts. Read more about ANVISA’s regulations from the experts here: Conducting Clinical Trials for Investigational Products in Brazil

