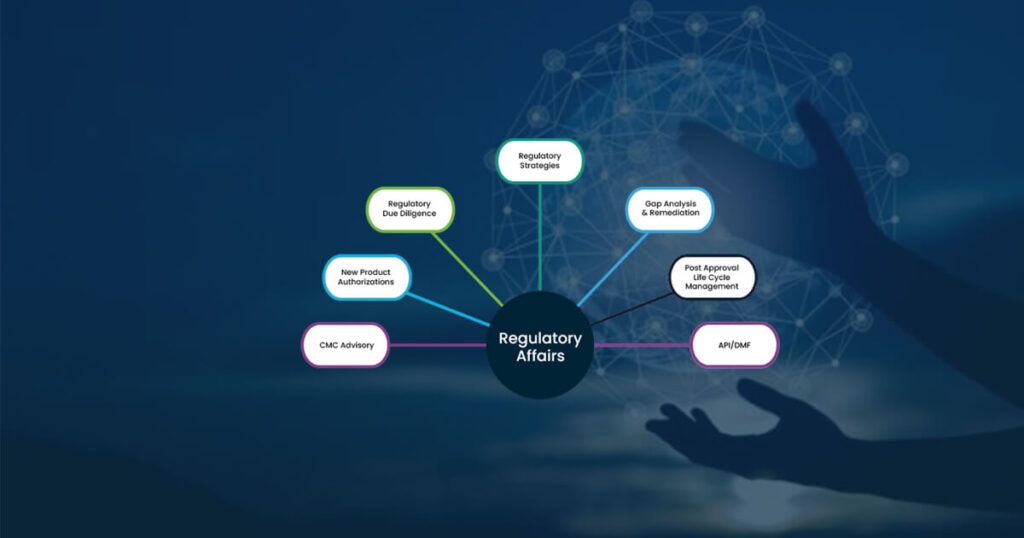
Regulatory Information Management Systems (RIMS)
Managing regulatory information for a pharmaceutical product along all stages of its life cycle is a key challenge in the pharmaceutical industry. Now, as more opportunity markets emerge bringing their own stringent regulations and changes in previous regulations, managing regulatory information is becoming increasingly complex which can delay market access for products. Furthermore, information is …
Regulatory Information Management Systems (RIMS) Read More »