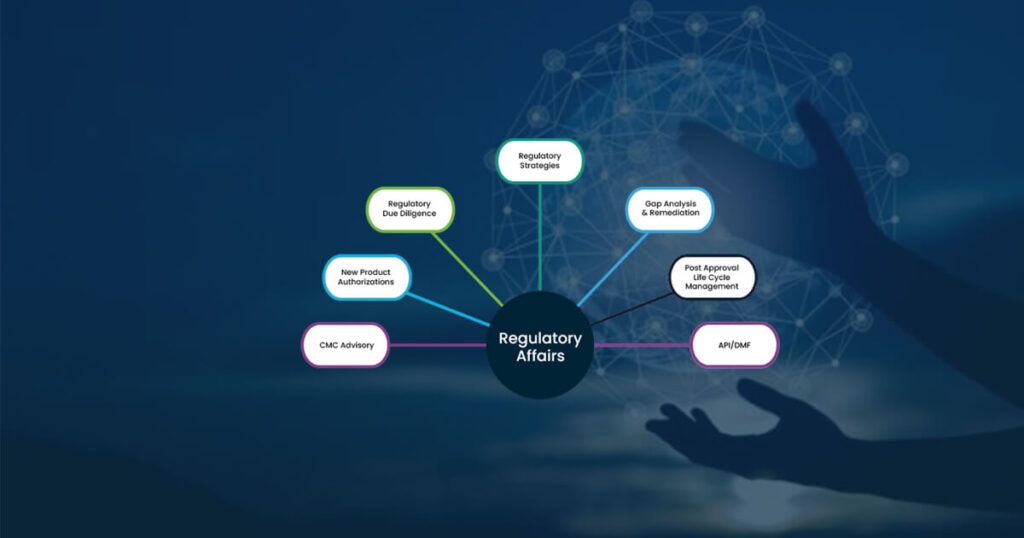
Role of Regulatory Affairs in the Pharmaceutical Industry
The pharmaceutical industry is highly regulated using legislations and regulations that bring together administrative, technical, and legal information about a medicinal product. These can be a combination of guidelines, procedures, policies, etc., that pharmaceutical organizations must adhere to, or follow, to get market approval for their products. This way, governments and their relative health authorities […]
Role of Regulatory Affairs in the Pharmaceutical Industry Read More »