Advanced Therapy Medicinal Products (ATMPs) are transforming the medical landscape, offering revolutionary treatments for diseases that previously lacked effective solutions. These products include cutting-edge innovations such as gene therapies, somatic cell therapies, and tissue-engineered products. Despite their potential, ATMPs face numerous challenges—scientific, regulatory, logistical, and financial—that hinder their development and global market access. Addressing these issues requires unprecedented collaboration among stakeholders and harmonization of regulatory frameworks across borders.
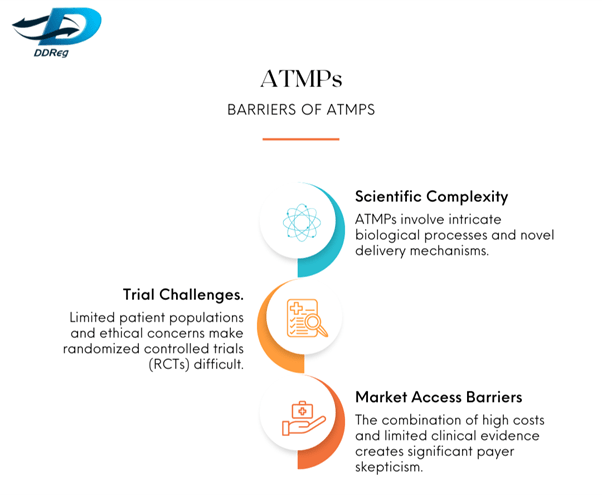
ATMPs have shown unparalleled potential in addressing high unmet medical needs, particularly in life-threatening conditions such as cancer, genetic disorders, and degenerative diseases. However, their complexity presents significant barriers:
Role of Collaboration in ATMPs
1. Partnerships Fueling Innovation
Collaboration between the public and private sectors has become the hallmark of ATMP development. Academic institutions and biotech firms often lead to early research while large pharmaceutical companies bring resources for late-stage development and commercialization of ATMPs. Notable examples are:
Novartis and University of Pennsylvania: Their partnership yielded Kymriah(r), the world’s first CAR-T therapy that is revolutionizing treatments for certain cancers.
Genethon: Genethon has played an essential role in pioneering gene therapies such as Zolgensma(r) to treat rare diseases.
By pooling their expertise and resources, these partnerships accelerate the journey from discovery to patient access.
2. Patient and Healthcare Provider Engagement
Patients and healthcare providers play an integral part in ATMP development, as patients provide invaluable insights about disease impact, trial design and recruitment/outcomes; such insights have even been integrated into decision-making processes by organizations like the European Medicines Agency‘s Committee for Advanced Therapies. Healthcare Providers need specialization training in ATMP administration as well as adverse event monitoring such as Cytokine Release Syndrome management.
Proactive engagement with these stakeholders ensures therapies are both effective and accessible.
3. Regulatory and HTA Collaborations
Early alignment with regulators and health technology assessment (HTA) bodies is critical. Initiatives like the EMA’s adaptive pathways pilot and parallel consultations streamline market access by reconciling evidence requirements. These collaborations reduce delays and help manufacturers anticipate and meet regulatory expectations.
Need for Harmonized Frameworks
Global harmonization is the cornerstone of ATMP success, addressing inefficiencies and promoting equitable access worldwide.
1. Aligning Regulatory Standards
Disparate regulations create roadblocks for ATMPs, particularly in multinational trials. For example:
- The EU’s hospital exemption rules vary across Member States, leading to inconsistent market access.
- Environmental risk assessments (ERAs) for gene therapies differ across regions, complicating compliance.
Unified standards would simplify these processes, reducing administrative burdens for developers.
2. Unified HTA Assessments
Fragmented HTA processes limit patient access and create disparities, yet collaborative HTA initiatives such as BeNeLuxA Initiative and EUnetHTA demonstrate its power. By pooling resources and expertise, these efforts streamline assessments while supporting fair pricing negotiations.
3. Global Patient Registries
Given the reliance of ATMPs on limited clinical evidence, robust post-market surveillance is essential. Coordinated global patient registries can:
- Facilitate long-term evidence collection.
- Support outcome-based payment models.
- Enable cross-comparisons of safety and efficacy.
Overcoming Barriers in ATMP development
Although collaboration and harmonization provide opportunities for significant advancement in ATMPs, several challenges still stand in the way of widespread adoption. Development costs tend to result in costly therapies, raising concerns regarding affordability and access. Further complicating matters, there may not be sufficient clinical data at approval time and require robust post-market studies which are either underfunded or inadequately conducted; misalignments between key stakeholders including regulators, payers, developers etc. can further lengthen approval/ reimbursement processes with delays impacting patient access resulting. These obstacles, however, present opportunities for progress.
Global initiatives, like the International Society for Cell and Gene Therapy, promote knowledge sharing across borders while innovative payment models such as installment-based pricing help balance financial sustainability with patient accessibility. By responding to challenges through strategic partnerships and forward-thinking solutions, ATMP landscape can continue to develop to ensure transformative therapies reach those most in need of transformational therapies.
Conclusion
ATMPs represent the future of medicine, offering groundbreaking therapies with the power to transform lives. But their full potential can only be realized through more than just scientific advancement; it requires an ecosystem where academia, industry, patients and healthcare providers all collaborate together in pushing innovation forward.
Harmonized regulatory and HTA frameworks that facilitate swift approval processes while guaranteeing global access are of equal significance. By working together and aligning systems, we can overcome existing obstacles to ATMP adoption worldwide and hasten its adoption faster. Together, we have the chance to redefine healthcare – making lifesaving therapies not just an aspiration but an accessible reality for patients everywhere.
DDReg Pharma specializes in providing tailored regulatory solutions to help you bring innovative therapies to patients worldwide. Reach out to our experts to discuss your regulatory needs. Read more from us here: Regulatory Landscape of Regenerative Medicines

