Since 2004, clinical trials (CTs) in the European Union (EU) have been governed by the Clinical Trials Directive (CTD) 2001/20/EC. This directive aims to harmonize the administrative requirements governing CTs across EU Member States. Each Member State (MS) has its own set of procedures, requirements, and timelines for CT operations. However, there was a need to streamline CT processes across the EU/EEA, enhance transparency, and generally improve CT efficiency to accelerate drug development leading to the transition of , the CTD to Clinical Trials Regulations (CTR) 536/2014.
Transition from CTD to CTR
The CTR officially came into effect on 31 January 2022, to simplify the process, increasing transparency, reducing administrative requirements, and streamlining the authorization process to expedite the CT initiation. The CTR introduces the Clinical Trials Information System (CTIS), a centralized portal for sponsors to register their CTs and submit related safety reports. It serves as a single point of access for both sponsors and regulatory authorities and makes it more efficient to conduct multinational CTs. Under the CTD, sponsors were required to submit separate CT applications to national competent authorities and ethics committees in each Member State in order to get approval for conducting a CT.
A 3-year transition period was set up to establish a smooth transition from CTD to CTR and sponsors were requested to either complete or transition to the new EU-CTR.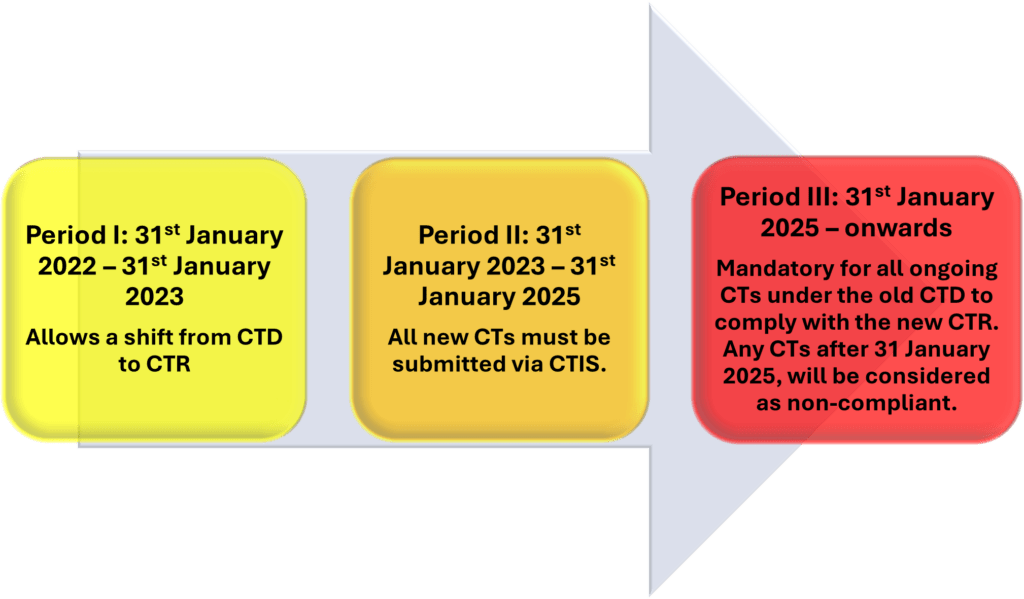
Strategies for maintaining compliance to safety reporting requirements in the CTD to CTR transition
The transition from the CTD to the CTR in the EU presents many changes to safety reporting requirements, highlighting the need for new strategies in order to maintain compliance. For example, one important strategy would be to implement streamlined, centralized safety data management systems that has the capacity to handle increased reporting complexity. Under the CTR, sponsors are required to report serious adverse events (SAE) and suspected unexpected serious adverse reactions (SUSARs) to the EU portal and database, enhancing transparency. Furthermore, establishing a dedicated team to monitor the updates in EU safety reporting regulations makes sure that processes are aligned with evolving guidelines.
Reporting SUSAR to Eudravigilance
SUSARs must be reported electronically using the EudraVigilance system. The data should be formatted according to the International Council for Harmonisation (ICH) E2B(R3) guidelines, which standardize the electronic transmission of individual case safety reports (ICSRs). The annual safety report must be submitted with yearly updates on the safety of each investigational medicinal product used in a clinical trial.
Before submitting SUSARs to EudraVigilance, sponsors must complete these steps:
- Obtain a Medical Dictionary for Regulatory Activities (MedDRA) license if needed or confirm if a fee waiver is applicable.
- Create an active EMA account via the EMA Account Management portal.
- Register the organization in the EMA’s Organisation Management Service (OMS) if not already registered.
- Assign a person responsible for EudraVigilance and request the “responsible person (RP)” role in EMA Account Management.
- Reporting Mode: (i)EVWEB: Use EudraVigilance to create and send SUSARs, (ii)Gateway: Send SUSARs from the sponsor’s database to EudraVigilance.
- Request User Roles: Obtain ‘EudraVigilance user roles’ through the EMA Account Management portal.
- User Training: Train users in EudraVigilance, ensuring at least one person per organisation completes the competency assessment or knowledge evaluation.
- Create Clinical Trial Application: Register a clinical trial in the Clinical Trials Information System (CTIS) or the European Union Drug Regulating Authorities Clinical Trials (EudraCT).
- Individuals unable to report SUSARs to EudraVigilance due to resource constraints may report them directly to the national competent authority of the Member State or EEA country where they occurred.
Conclusions
With the introduction of the EU Clinical Trials Regulation (EU-CTR), the regulatory landscape for clinical trials has become more streamlined, harmonized, and transparent. This regulation aims to facilitate the multinational clinical trial process, ensuring the protection of trial participants and the integrity of data. All clinical trials in the EU must be completed or transitioned to the new EU-CTR framework by 1 January 2025. Sponsors now face a limited timeframe to ensure their trials comply with this updated regulatory framework. The new regulation establishes a single access point for clinical trial applications through the Clinical Trials Information System (CTIS) portal. This enables sponsors to submit a single dossier for all EU member states, reducing administrative burdens and expediting the assessment process. As the EU-CTR is fully implemented, staying informed and leveraging available resources will be crucial for achieving and maintaining compliance in clinical trials.
In addition to providing regulatory and pharmacovigilance services for global customers, DDReg Pharma offers specialized clinical regulatory services to assist with Clinical Trial Applications in Europe. For further information, connect with our experts. Read more from the experts about the new Clinical Trial Regulations here: Accelerating Clinical Trials in the European Union

